GMP violations are found in some pharmaceutical manufacturing facilities in India. These GMP violations mean these manufacturers do not follow the good manufacturing practices regulations and this may cause serious health problems to the patient consuming the drug products manufactured in these facilities.
When any facility does not follow the GMP regulation, it is considered a GMP violation. One the common GMP violations is incorrect labeling, when all ingredients of the product are not listed properly on the label. Sometimes contaminated products are distributed for sale or products are not tested properly before releasing the product into the market.
Related: Consequences of GMP Violation
Senior management should also have a keen eye on the implementation of GMP regulation and must visit the manufacturing area at regular intervals. Involvement of the senior management in implementation of GMP regulation will motivate the junior staff to follow the recommendations.
Causes of GMP Violations
GMP regulations are recommendations to ensure the safety and effectiveness of pharmaceutical products. GMP regulation ensures the effectiveness and safety of the products and it also ensures that the product does not have any harmful ingredients.When any facility does not follow the GMP regulation, it is considered a GMP violation. One the common GMP violations is incorrect labeling, when all ingredients of the product are not listed properly on the label. Sometimes contaminated products are distributed for sale or products are not tested properly before releasing the product into the market.
Related: Consequences of GMP Violation
Who is Responsible for GMP Violations
To follow the GMP regulation is not the responsibility of any individual in the facility but it is a collective effort from junior staff to senior management. All departments need to play their important role to comply with the GMP regulations. Quality assurance is there to ensure the product quality there for the quality assurance head is mainly responsible for any GMP violation. Quality control also plays an important role in maintain the product quality so the person analyzing the product is also responsible for the production of quality compromised products.
Senior management should also have a keen eye on the implementation of GMP regulation and must visit the manufacturing area at regular intervals. Involvement of the senior management in implementation of GMP regulation will motivate the junior staff to follow the recommendations.
To minimize the chances of GMP violations in the facility a strong quality management system must be implemented and employees must be trained properly to carry their duties. If anyone in the facility experience any GMP violation, he/she must speak to the plant manager or quality assurance head to in order to resolve the issue. To find the GMP issues all employees of the company should be well trained and they must know all GMP recommendations.
Poor quality control and ineffective safety monitoring system is the main reason for the GMP violation in Indian pharmaceutical manufacturing facilities. Sometimes inadequate manufacturing procedures are followed means the manufacturing process is deviated or some parts of the process are skipped due to which a low-quality product is produced.
Related: Data Integrity - A Major Problem in Pharmaceuticals
Additionally, government regulators are often unable to force companies to follow GMP regulations. As a result, unethical businesses can continue to run without fear of punishment. These problems have a significant impact on the quality of the Indian pharmaceutical industry. In past few years, the Indian government has been reforming its regulatory system and enhancing its enforcement capabilities. However, it will take years to get results until then companies in India will continue to face the challenges to comply with the GMP requirements.
How do GMP Violations Affect Manufacturers in India?
If there are a lot of GMP violations happen, this can have a big impact on pharmaceutical industry business in India. GMP violations can hurt pharmaceutical companies in four ways.
1. Production Suspension: GMP violation can lead to the suspension of the production if violation is serious. It means company could lose the sales and revenue.
2. Fines: Regulators may fine the company for violating the GMP regulations and the amount can be up to the millions of dollars. This can cause financial instability for the company.
4. Damage to Reputation: GMP violation can result in negative publicity for the company and customer can avoid the purchase the drug products.
GMP violations are serious issues because this is a concern for human life. The situation in India is improving day by day, the government is working hard to implement the rule and regulations. If the situation does not change then it will be difficult to do pharmaceutical business in India in the coming time. It will be very interesting to see the future of the pharmaceutical business and its growth in India.
Reasons for GMP Violations in Indian Facilities
It does not mean that all Indian facilities have GMP issues, we are talking only about the facilities having issues. India has 10,000+ pharmaceutical manufacturing facilities and GMP violation issues are found in less than 1% of facilities.Poor quality control and ineffective safety monitoring system is the main reason for the GMP violation in Indian pharmaceutical manufacturing facilities. Sometimes inadequate manufacturing procedures are followed means the manufacturing process is deviated or some parts of the process are skipped due to which a low-quality product is produced.
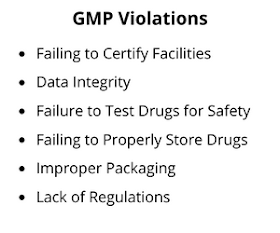
Common GMP Violations found at Indian facilities
As per the GMP inspections by different regulatory agencies more than 100 types of GMP violations are found in Indian pharmaceutical manufacturing facilities. Some most frequently found violations are as follows.1. Data Integrity
Data integrity issues are the most common GMP violations found in Indian pharmaceutical manufacturing facilities. Data missing and data manipulation are commonly found data integrity issues. In some companies data falsification is also observed by the regulatory agencies where false analytical data was created to release the product.Related: Data Integrity - A Major Problem in Pharmaceuticals
2. Procedures not in Writing or Fully Followed
It is also very common where the procedure followed in quality control are not written in SOPs and STPs. In some other cases written procedures are not followed by the personnel working in the quality control or manufacturing.3. Cleaning and Sanitization
Equipment and utensils are not cleaned, maintained or sanitized at appropriate intervals to prevent contamination that would alter the safety, identity, strength, quality or purity of the drug product. Area cleaning and sanitization are also found unsatisfactory in some facilities.4. Failing to Properly Store Drugs
Proper drug storage is essential for safety of the drug products. Some facilities are failed to maintain the temperature and humidity required for the storage of drug products. It can decrease the effectiveness of the product and cause the degradation of the active ingredient.5. Improper Packaging
Improper packaging is another violation found in drug manufacturing facilities. In some cases low quality packaging materials are used or product is not properly labeled. In other some cases packaging materials are not sampled, examined, or tested upon receipt and before use in packaging and labeling of a drug product.6. Failure to Investigate Discrepancies
There is a failure to thoroughly review or investigate any incidence or any unexplained discrepancy whether the batch has been already distributed. Some companies failed to implement the proper corrective and preventive actions.Key Points to Avoid GMP Violations
GMP violations can be avoided very easily by keeping the following key points in mind.- Follow all guidelines provided by regulatory bodies.
- Make sure all processes are in line with GMP requirements.
- Ensure products are safe and effective.
- Keep records of procedures and activities to ensure compliance is maintained.
- Labels are a primary tool for identifying GMP, label requirements include wording, location and format for GMP compliance.
- Labels must contain all necessary GMP information to ensure compliance.
- compliant procedures and monitoring.
- Production records are needed to monitor GMP (minimum efficient scale).
- Proper disposal of by-products must be planned in advance.
- Producers must ensure processed components do not compromise GMP protocols.
- Experts in this regulatory area should verify GMP requirements and current regulations.
- Traceability is a great concern because it ensures that raw materials are used properly and their safety monitored before being added to any product.
Why are these cGMP Violations Taking Place at Indian Facilities?
In recent years GMP violations in Indian pharmaceutical companies are increased drastically, it is due to the increase in export in past few years. Some companies do not take the drug efficiency and safety seriously. This casual approach does not help to create a quality culture.Additionally, government regulators are often unable to force companies to follow GMP regulations. As a result, unethical businesses can continue to run without fear of punishment. These problems have a significant impact on the quality of the Indian pharmaceutical industry. In past few years, the Indian government has been reforming its regulatory system and enhancing its enforcement capabilities. However, it will take years to get results until then companies in India will continue to face the challenges to comply with the GMP requirements.
How do GMP Violations Affect Manufacturers in India?
If there are a lot of GMP violations happen, this can have a big impact on pharmaceutical industry business in India. GMP violations can hurt pharmaceutical companies in four ways.
1. Production Suspension: GMP violation can lead to the suspension of the production if violation is serious. It means company could lose the sales and revenue.
2. Fines: Regulators may fine the company for violating the GMP regulations and the amount can be up to the millions of dollars. This can cause financial instability for the company.
4. Damage to Reputation: GMP violation can result in negative publicity for the company and customer can avoid the purchase the drug products.
GMP violations are serious issues because this is a concern for human life. The situation in India is improving day by day, the government is working hard to implement the rule and regulations. If the situation does not change then it will be difficult to do pharmaceutical business in India in the coming time. It will be very interesting to see the future of the pharmaceutical business and its growth in India.
Get editable audit documentsView List

Eye opener observations on lack of GMP compliance in the Indian pharmaceutical industry. We must try to rectify the defiencies as soon as possible in collaboration with Government agencies.
ReplyDelete