Catecholamine biosynthesis is divided into three stages:
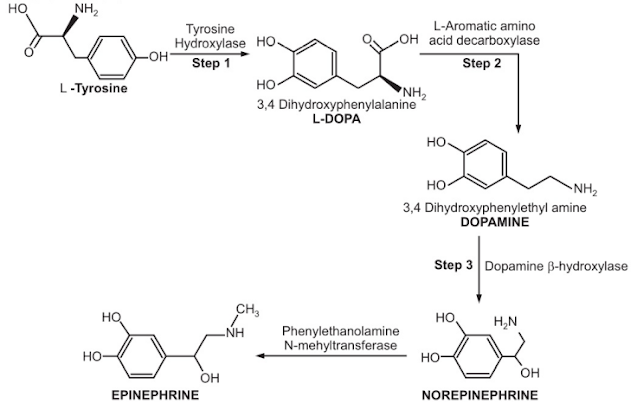
The biosynthesis of norepinephrine is a three-step process -
Step 1 - tryosine hydroxylase catalyzes the conversion of L - tryosine to L - dihydroxyphenylalanine (L - DOPA). This is the most time-consuming stage in catecholamine biosynthesis. Alpha methyltryosine inhibits tryosine hydroxylase (metyrosine).
Step 2 - solution of (L-DOPA) catalyzed by L-aromatic amino acid decarboxylase (DOPA decarboxylase) and blocked by methyldopa.
Step 3 - selective hydroxylation of dopamine to create NE catalyzed by dopamine beta hydroxylase, which is blocked by disulfiram.

MOA works by oxidatively deaminating catecholamines.
R – CH2 - NH2 --> R – CH = NH --> R – CHO + NH4
COMT nearly primarily methylates the meta hydroxyl group of catecholamines. The diagram below depicts the several metabolic pathways of catecholamines (norepinephrine and epinephrine) -

In humans, vanillylmandelic acid (VMA) is an important consequence of norepinephrine and epinephrine metabolism. It is first converted to DHPG by monoamine oxidase and then to normetanephrine by catechol-O-methyltransferase. Furthermore, MHPG is a significant norepinephrine metabolite generated primarily by COMT's O-methylation of DHPG. Deamination of metanephrine can also result in the formation of MHPG.
Drugs that work on the adrenergic nerve system, with a few exceptions, all include some chemical constituents of the endogenous agonist, epinephrine. Natural catecholamines include adrenaline, norepinephrine, and dopamine. They are in charge of the majority of the "flight or fight" reactions.
Norepinephrine is a neurotransmitter found in the sympathetic nervous system as well as the brain. It also acts as a precursor for adrenaline production in the adrenal gland. Adrenaline is a hormone produced by the adrenal medulla. In 1895, adrenal extracts were shown to have a pressor action. Abel and Crawform (1897) were the first to isolate it from the adrenal medulla in the form of its polybenzoyl derivative. Abel named the active principle adrenaline. One of the laboratories involved in the identification and purification of this pressor ingredient (Japanese scientist Takamine) dubbed it adrenaline. Stoltz synthesized it for the first time in 1904.
Catecholamines are easily oxidized to create ortho quinone-like molecules. The appearance of a pink to brown colour is a sign of oxidative degradation. As a result, antioxidants such as ascorbic acid or sodium bisulfite may be employed to stabilize catecholamine solutions.
In 1910, Barger and Dale produced and tested a significant number of synthetic amines that were structurally similar to epinephrine. They labelled these substances' action as sympathomimetic. Tullar solved the racemic mixing that resulted in 1948. Dextro-epinephrine was found to be roughly one-twelfth as powerful as laevo-epinephrine after resolution. Elliott suggested its role as a neurotransmitter in the sympathetic nervous system in 1904.

- Central nervous system adrenergic and dopaminergic neurons
- The adrenal medulla.
- Sympathetic in autonomic nervous system neurons.
The biosynthesis of norepinephrine is a three-step process -
Step 1 - tryosine hydroxylase catalyzes the conversion of L - tryosine to L - dihydroxyphenylalanine (L - DOPA). This is the most time-consuming stage in catecholamine biosynthesis. Alpha methyltryosine inhibits tryosine hydroxylase (metyrosine).
Step 2 - solution of (L-DOPA) catalyzed by L-aromatic amino acid decarboxylase (DOPA decarboxylase) and blocked by methyldopa.
Step 3 - selective hydroxylation of dopamine to create NE catalyzed by dopamine beta hydroxylase, which is blocked by disulfiram.
Catabolism of catecholamine
Monoamine oxidase (MAO) and catechol O-methyltransferase (CAT) are the two main enzymes involved in catecholamine metabolism (COMT). The MAO enzyme metabolizes intraneuronal catecholamines, whereas the COMT enzyme predominantly acts on catecholamines that enter the blood and extraneuronal tissues.MOA works by oxidatively deaminating catecholamines.
R – CH2 - NH2 --> R – CH = NH --> R – CHO + NH4
COMT nearly primarily methylates the meta hydroxyl group of catecholamines. The diagram below depicts the several metabolic pathways of catecholamines (norepinephrine and epinephrine) -
In humans, vanillylmandelic acid (VMA) is an important consequence of norepinephrine and epinephrine metabolism. It is first converted to DHPG by monoamine oxidase and then to normetanephrine by catechol-O-methyltransferase. Furthermore, MHPG is a significant norepinephrine metabolite generated primarily by COMT's O-methylation of DHPG. Deamination of metanephrine can also result in the formation of MHPG.
Drugs that work on the adrenergic nerve system, with a few exceptions, all include some chemical constituents of the endogenous agonist, epinephrine. Natural catecholamines include adrenaline, norepinephrine, and dopamine. They are in charge of the majority of the "flight or fight" reactions.
Norepinephrine is a neurotransmitter found in the sympathetic nervous system as well as the brain. It also acts as a precursor for adrenaline production in the adrenal gland. Adrenaline is a hormone produced by the adrenal medulla. In 1895, adrenal extracts were shown to have a pressor action. Abel and Crawform (1897) were the first to isolate it from the adrenal medulla in the form of its polybenzoyl derivative. Abel named the active principle adrenaline. One of the laboratories involved in the identification and purification of this pressor ingredient (Japanese scientist Takamine) dubbed it adrenaline. Stoltz synthesized it for the first time in 1904.
Catecholamines are easily oxidized to create ortho quinone-like molecules. The appearance of a pink to brown colour is a sign of oxidative degradation. As a result, antioxidants such as ascorbic acid or sodium bisulfite may be employed to stabilize catecholamine solutions.
In 1910, Barger and Dale produced and tested a significant number of synthetic amines that were structurally similar to epinephrine. They labelled these substances' action as sympathomimetic. Tullar solved the racemic mixing that resulted in 1948. Dextro-epinephrine was found to be roughly one-twelfth as powerful as laevo-epinephrine after resolution. Elliott suggested its role as a neurotransmitter in the sympathetic nervous system in 1904.
Get subject wise printable pdf notesView Here


No comments:
Post a Comment
Please don't spam. Comments having links would not be published.