Baeyer synthesized acetylcholine for the first time in 1867. In general, activation of the parasympathetic nervous system causes pupillary and bronchial constriction, a decrease in heart activity, and an increase in digestive system activity, i.e., salivation and other GIT secretions.
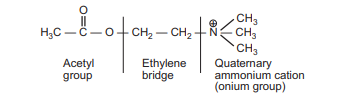
The following are some of the most important chemical properties of the acetylcholine molecule:

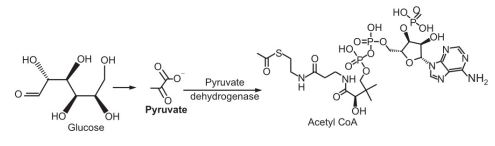
Choline acetyl transferase is an enzyme that catalyzes the production of acetyl choline from choline and acetyl coenzyme A. Acetyl CoA is synthesized from pyruvate in the cytosol via the pyruvate dehydrogenase process. Choline is converted from the amino acid serine to aminoethanol. Choline is formed when aminoethanol is trimethylated.
The enzyme's basic unit is a tetramer with a molecular weight of 320,000; each protomer has an active site. Normally, three of these tetrameric units are joined to a 50 2 nm stem by disulphide bonds. The enzyme's amino acid composition resembles the acetylcholine receptor in its large number of acidic amino acids, according to an analysis of its amino acid composition.

The following are some of the most important chemical properties of the acetylcholine molecule:
- It is an amino alcohol that is chemically an ester of acetic acid and choline.
- On a structural level, it provides three molecular modification sites:
- Ethylene bridge
- Quaternary ammonium group
- Acetyl group
- An ethylene bridge connects the quaternary ammonium group (i.e., onium group) to an ester group.
- Acetylcholine is stable in acidic solutions but unstable in alkaline solutions.
- The cholinesterase enzyme quickly hydrolyzes free acetylcholine in tissue fluids and circulation to acetic acid and choline molecule.
- Some of acetylcholine's activities are mediated by G-protein coupled muscarinic receptors, whereas others are mediated by nicotinic receptors.
Choline acetyl transferase is an enzyme that catalyzes the production of acetyl choline from choline and acetyl coenzyme A. Acetyl CoA is synthesized from pyruvate in the cytosol via the pyruvate dehydrogenase process. Choline is converted from the amino acid serine to aminoethanol. Choline is formed when aminoethanol is trimethylated.
Catabolism of Acetylcholine
Acetylcholinesterase is an enzyme that breaks down acetylcholine into choline and acetate after it is released in the synaptic cleft. Acetylcholinesterase does not destroy Ach stored in vesicles.Metabolism of Acetylcholine
The free acetylcholine in the blood and other tissues is rapidly hydrolyzed by either e-cholinesterase (found in erythrocytes) or s-cholinesterase (present in the serum). Dale (1914) was the first to suggest the notion of acetylcholine enzymatic breakdown in the blood and other tissues. Butyrocholinesterase is another name for serum cholinesterase, while acetylcholinesterase is another name for e-cholinesterase. Cholinesterase are non-selective enzymes. Some of the features of e-cholinesterase are shared by a variety of different s-cholinesterase. Both of these kinds hydrolyze a high number of choline and other carboxylic acid esters.The enzyme's basic unit is a tetramer with a molecular weight of 320,000; each protomer has an active site. Normally, three of these tetrameric units are joined to a 50 2 nm stem by disulphide bonds. The enzyme's amino acid composition resembles the acetylcholine receptor in its large number of acidic amino acids, according to an analysis of its amino acid composition.
Get subject wise printable pdf notesView Here



No comments:
Post a Comment
Please don't spam. Comments having links would not be published.