Theory of indicators - Titrations are evaluated by determining the endpoint of the titration with the aid of an indicator, which indicates that the titration has been completed. Titrating acids and bases is generally carried out using weak acids or weak bases as indicators. The color of these substances changes with pH. Several common indicators have been listed below, along with the pH range for each:
Two theories account for acid-base indicators' pH-dependent color changes.
The theory of Ostwald: The theory states
a) The acid-base indicator is ionized, resulting in a color change. The ionized indicator has a different color than the unionized indicator.
(b) As acids and bases are either weak acids or weak bases, the indicator has the potential to ionize. Since it is common to have H+ ions in acids, the indicator's ionization is very low when it is a weak acid, but it is fairly ionized in Alkalies. OH- ions exist in both acids and alkalis so ionization in a weak base is greater in acids and much lower in alkalis.
Phenolphthalein - Hugh is the chemical symbol for phenolphthalein. In solution, Phenolphthalein ionizes as follows:
Hugh ↔ H+ + Ph
Colorless turns into Pink color
Applying law of mass action,
K = [ H+ ][Ph- ] / [ H ]
Pink ions are formed by phenyl groups, while colorless undissociated molecules are created by phenolphthalein. With an acid present, Hugh ionization is negligible due to a large concentration of hydrogen ions leading to a shift to the left-hand side of the equilibrium. This results in a colorless, clear solution. Alkali causes hydrogen ions to be removed by OH- ions, resulting in a shift in equilibrium to the right. This occurs due to the high concentration of Ph- ions in the solution, which causes the solution to turn pink.
In an acidic medium, phenolphthalein exists in benzoic form, and, therefore, is colorless, while it exists in quinonoid form, which gives it its pink color in an alkaline medium.

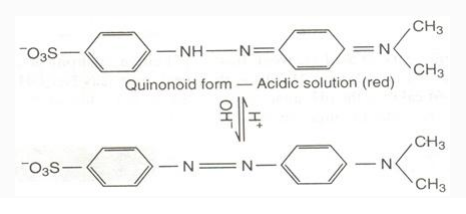
When methyl orange is in an acidic solution, the quinonoid form is formed and when it is in alkaline solution, the benzenoid form is formed. The colors of benzenoid and quinonoid forms are yellow and red, respectively.
Classification of acid-base titrations
Titration experiments are used to gather information about the composition of a solution containing acid or base. An acidic or basic fingerprint can be determined by titration for hundreds of compounds, both organic and inorganic. Titration between acids and bases occurs when acids are titrated with bases. The endpoint is usually determined by adding indicators.
A titration of acid-base determines whether acid or base is present. You can use a pH-based titration to determine the following.
Two theories account for acid-base indicators' pH-dependent color changes.
The theory of Ostwald: The theory states
a) The acid-base indicator is ionized, resulting in a color change. The ionized indicator has a different color than the unionized indicator.
(b) As acids and bases are either weak acids or weak bases, the indicator has the potential to ionize. Since it is common to have H+ ions in acids, the indicator's ionization is very low when it is a weak acid, but it is fairly ionized in Alkalies. OH- ions exist in both acids and alkalis so ionization in a weak base is greater in acids and much lower in alkalis.
Phenolphthalein - Hugh is the chemical symbol for phenolphthalein. In solution, Phenolphthalein ionizes as follows:
Hugh ↔ H+ + Ph
Colorless turns into Pink color
Applying law of mass action,
K = [ H+ ][Ph- ] / [ H ]
Pink ions are formed by phenyl groups, while colorless undissociated molecules are created by phenolphthalein. With an acid present, Hugh ionization is negligible due to a large concentration of hydrogen ions leading to a shift to the left-hand side of the equilibrium. This results in a colorless, clear solution. Alkali causes hydrogen ions to be removed by OH- ions, resulting in a shift in equilibrium to the right. This occurs due to the high concentration of Ph- ions in the solution, which causes the solution to turn pink.
Quinonoid theory
This theory states:
There are two types of tautomeric acid-base indicators with different structures. The two forms are in equilibrium. Two types of compounds can be found in nature: benzenoid and quinonoid.
The two forms of the solution differ in color. One of the tautomeric forms is interconverted into another, causing the color change.
The acidic one is the predominant form, while the other is alkaline.
In an acidic medium, phenolphthalein exists in benzoic form, and, therefore, is colorless, while it exists in quinonoid form, which gives it its pink color in an alkaline medium.
When methyl orange is in an acidic solution, the quinonoid form is formed and when it is in alkaline solution, the benzenoid form is formed. The colors of benzenoid and quinonoid forms are yellow and red, respectively.
Classification of acid-base titrations
Titration experiments are used to gather information about the composition of a solution containing acid or base. An acidic or basic fingerprint can be determined by titration for hundreds of compounds, both organic and inorganic. Titration between acids and bases occurs when acids are titrated with bases. The endpoint is usually determined by adding indicators.
A titration of acid-base determines whether acid or base is present. You can use a pH-based titration to determine the following.
- Acid or base concentration
- will indicate how strong or weak it is.
- Acidic pKa or base pKb of an unknown compound.
- A strong acid combines with a strong base or you can say strong acid and a strong base
- A weak acid reacts with a strong base
- A reaction where bases are weak and acids are strong
- A reaction where acids are weak and bases are weak
Get subject wise printable pdf documentsView Here

No comments:
Post a Comment
Please don't spam. Comments having links would not be published.