Introduction to Pharmaceutical Dosage Form
Drugs are rarely delivered as pure chemical entities but are approximately usually provided as prepared formulations i.e. dosage form. After converting them into an appropriate dose formulation, they are delivered in several dosage forms.To create an alternative dosage form, non-medicinal chemicals (also known as pharmaceutical ingredients or excipients) are added. By adding pharmaceutical ingredients that solubilize or suspend or thicken or dilute or emulsify or stabilize or preserve them, drug dosage forms can be made more effective and appealing.
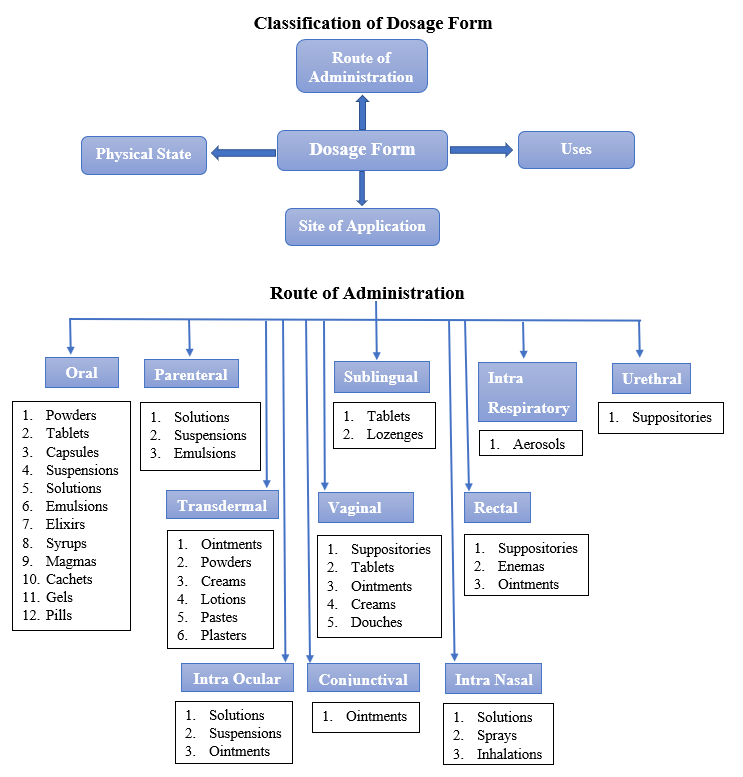
Definition of Dosage Form
Dosage forms are the mechanism by which drug molecules / APIs are administered to areas of action inside the body to generate maximum intended benefits and the lowest unwanted effects.OR
The Dosage form is the combination of Active Pharmaceutical Ingredients (API) and Excipients in the formulation.
Need of Dosage Forms
Mainly depend on Patient Safety and Drug Safety/ Benefits.1. Deliver precise dosages in a safe and easy manner. Example – Tablets, capsules, syrups
2. Cover bitter taste or odor of drug substances. Example – Capsules, coated tablets, flavored syrups
3. Insoluble or unstable in the selected vehicle, provide a liquid formulation of the insoluble or unstable medication. Example – Suspension
4. Controlled-release methods prolong the duration of medication effect. Example – Controlled release tablets, capsules, suspensions
5. After oral delivery, a drug substance is protected from stomach acid. Example – Enteric-coated tablets
6. Provide optional drug action from topical administration sites. Example – Ointment, cream, ear and nasal preparations
7. Drugs can be injected into the body's tissues. Example – Implants
8. Inhalation treatment is the most effective way to get optimum medication activity. Example – Inhalants
9. Liquid dosage forms of chemicals soluble in the vehicle of your choice. Example – Solution
10. Provide for the introduction of medication into the body’s orifice. Example – Rectal and vag*inal suppositories
11. Protection of a drug substance from atmospheric oxygen or moisture. Example – Coated capsules, sealed ampules.
Definition of Drug (Active Pharmaceutical Ingredients)
Drogue is an old French term that means "dry herb" and is sometimes used interchangeably with the word "drug".Chemical compounds intended for use in the diagnosis, prevention, treatment, and management of disease(s) in humans and other animals are referred to as "pharmaceutical products" or "pharmaceuticals". Chemical/organic synthesis, molecular modification, and biotechnology have all been used to produce medicines in recent years.
OR
The Active Pharmaceutical Ingredient (API) is the component of a medication that creates its action.
Definition of Excipients
- Do not increase or affect the therapeutic action of the active components.
- They are also known as inactive components or excipients and have no pharmacological action in general.
- Examples of inactive components are dyes, preservatives, sweetening agents, binding materials, coloring agents and flavoring agents, etc.
Definitions of Different Dose Forms
1. Liquid
- Droughts: Liquid oral formulations comprising single or several doses of medication.
- Elixirs: Excipients and medicaments in a liquid formulation for oral administration.
- Emulsions: Water-based suspension of oils and fats using an emulsifying agent. Emulsifying agent coats oil particles so they do not coalesce when the interfacial tension between oil and water decreases. As a result, an emulsion is created.
- Suspensions: One or more active components dispersed in a suitable medium are used in biphasic liquid formulations for oral administration. When shaken, it disperses into a uniform suspension that is stable enough to deliver the precise dosage.
- Gargles: Externally applied aqueous solutions that are concentrated for treating throat infections.
- Gels: Dispersions of medicaments in water used as antacids.
- Lotions: External liquid preparations are generally administered without friction.
- Liniments: The application of external liquid preparations is generally done via friction.
- Mixtures: One or more medications are included in liquid oral preparations.
- Mouthwashes: In a similar manner to gargles, these mouthwashes are used for oral cleanliness and to treat oral infections.
- Nasal drops: Dropper-instilled liquid solutions used to treat nose infections and blockages.
- Solutions: Liquid medicine that can be used for internal or exterior applications.
- Syrups: With or without sugar and medicaments, sweet, viscous, concentrated liquid medicines are made.
2. Solid
- Powders: Solid dose formulations comprising micron-sized, finely fragmented particles.
- Tablets: Medication in solid dose form, either with or without excipients.
- Granules: Particles in a group.
- Capsules: Gelatin capsules are used to encapsulate drugs.
- Pills: Excipients are contained in this small pill.
- Lozenges: Sugar and gum-based solid formulations used to treat mouth and throat disorders.
- Suppositories: Solid dosage form carrying medication that is put into bodily cavities other than the mouth, such as the rectum, nose, or ear.
3. Semisolid
- Ointments: Ointment-based semisolid dose forms for external application that include or do not contain medications.
- Creams: With or without medicaments, semisolid external dose forms with an appropriate fatty basis are available.
- Paste: With an appropriate fat basis, semisolid external dosage forms include a significant proportion of finely powdered medicaments.
- Gels: Contains hydrophilic or hydrophobic base and gelling agents. Transparent semisolid dose forms for external usage.
4. Gaseous
- Aerosols: Dispersion of solid or liquid particles in gas for application to the respiratory tract, using an atomizer.
- Inhalations: It consists of pharmaceutical liquid preparations for internal consumption, which are either dispersed or suspended in the propellant.
- Sprays: Application of alcohol-containing medication aerosols to the nose or throat using an atomizer or nebulizer.
Frequently Asked Questions on Pharmaceutical Dosage Forms
Q: What are pharmaceutical dosage forms?
Pharmaceutical dosage firms are the physical forms of medicines that are formulated for administration to patients for treatment. Tablets, capsules, syrups, suspensions, creams, ointments, inhalers, injections and patches are some commonly used dosage forms.
Q: Why are different dosage forms used in pharmaceuticals?
Different dosage forms are used to ease the administration of the drug. It includes the patient needs, product stability and efficiency of the drug. The formulation of the drug dosage form is chosen on the basis of the requirement of the route of drug administration, drug release profile, patient convenience and characteristics of the active pharmaceutical ingredient.
Q: What factors are considered when selecting a suitable dosage form?
Several factors are considered while selecting the dosage forms including the age and condition of the patient, route of administration, property of the drug substance and stability of the drug product. The dosage form should ensure proper absorption, delivery and therapeutic effect.
Q: What are the advantages of solid dosage forms (tablets and capsules)?
Solid oral dosage forms like tablets and capsules have several advantages including stability of the product, accurate dosing and easy administration. These can be formulated to release immediately, delayed or sustained release. Tables may have multiple active ingredients to provide combination therapy that is not possible with other dosage forms.
Q: What are the advantages of liquid dosage forms (syrups, solutions, suspensions)?
Liquid dosage forms are easy to swallow and have faster absorption and flexibility of dosing. They are suitable for elderly people and children, those who can't take solid dosage forms. These are formulated with flavors that improve the taste of the medicine. The dose can be adjusted easily for different age groups.
Q: What are the advantages of topical dosage forms (creams, ointments, gels)?
Topical dosage forms are applied on the skin and have advantages like target delivery, reduced side effects and easy application. Topical dosage forms are used for dermatological conditions, pain relief and wound healing.
Q: What are the advantages of parenteral dosage forms (injections)?
Parenteral or injectable dosage forms are administrated intravenously, intramuscularly, subcutaneously or intradermally and selection from these is depend upon the requirement of treatment. These have many advantages including rapid and precise drug delivery and the ability to administer the drug in high potency and critical conditions when the patient is not able to take other dosage forms.
Q: What are the considerations for developing novel dosage forms?
In the formulation of novel dosage forms pharmaceutical scientists consider many factors including drug solubility, bioavailability, stability, drug release kinetics, route of drug administration, manufacturing feasibility, regulatory requirements and intellectual property rights. These all are done step by step.
Q: How are pharmaceutical dosage forms evaluated for quality and performance?
After manufacturing all dosage forms undergo various quality checks to ensure the quality and safety of the product including content uniformity, dissolution test, stability of product, microbiological quality and compatibility with packing material. These all tests are done according to the guidelines of regulatory authorities to ensure that the product meets the established specifications.
Q: Are there any limitations or challenges associated with pharmaceutical dosage forms?
Pharmaceutical dosage forms have certain limitations and challenges including product stability issues, variation in the bioavailability of the drug, difficulty in the formulation of certain drugs, storage and handling and cost of the final product.
Get subject wise printable pdf documentsView Here


Easy understanding
ReplyDeleteNeed to add Oral thin strips in solid sublungual and transdermal patches in transdermal dosage forms
ReplyDeleteNice
ReplyDeleteNice performance
ReplyDelete