When you subscribe to a new internet plan, you tend to check the download speed by downloading something in order to verify if you are receiving the promised data rate. But are the data rates consistent throughout the day? Do you get the same speed if another device is also connected to the network? These are the kind of questions that are answered by performance qualification.
For most pharmaceutical equipment, the operational qualification phase is usually a walk in the park. In the absence of any form of load, it is very easy to provide the promised specifications. However, performance qualification is what truly determines how viable a piece of equipment is. Suppose a tablet press passes the operational qualification phase with flying colors.
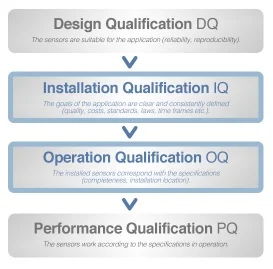 But it fails to deliver the same pressure rating with a few grams of powder in it. Or consider a homogenizer, though performing seamlessly when empty, provides only a fraction of the specified rpm with a few hundred kilos of the load in it.
But it fails to deliver the same pressure rating with a few grams of powder in it. Or consider a homogenizer, though performing seamlessly when empty, provides only a fraction of the specified rpm with a few hundred kilos of the load in it.
 But it fails to deliver the same pressure rating with a few grams of powder in it. Or consider a homogenizer, though performing seamlessly when empty, provides only a fraction of the specified rpm with a few hundred kilos of the load in it.
But it fails to deliver the same pressure rating with a few grams of powder in it. Or consider a homogenizer, though performing seamlessly when empty, provides only a fraction of the specified rpm with a few hundred kilos of the load in it.
Without performing the performance qualification phase, a piece of equipment might completely fail to deliver any product. But even worse, unknown to the manufacturer, it might produce an undesired product. If the manufacturer does manage to notice that the final product is flawed, then the organization incurs massive losses because of the raw materials wasted on the discarded batch and also loses valuable time, which is as bad as incurring monetary losses, in today's cutthroat market.
If the manufacturer fails to notice and releases the imperfect batch into the market, it exposes them to customer dissatisfaction and massive lawsuits. Now that the importance of performance qualification has been established, we come to the question of whose responsibility it is to perform it.
Related: Installation Qualification (IQ) in Pharmaceuticals
Related: Installation Qualification (IQ) in Pharmaceuticals
The pharmaceutical companies, or the various companies manufacturing the equipment? The obvious answer is the equipment manufacturers, right? Not only do they have an ethical obligation to perform performance qualification, but it is essential for providing the clients with the specifications they demand. But the reality is, performance qualification should be performed by both the equipment manufacturers as well as their clients, the pharmaceutical companies.
Related: Design Qualification (DQ) of Equipment
Related: Design Qualification (DQ) of Equipment
Performance qualification is a part of equipment validation process and there are a number of reasons why pharmaceutical plants should perform it. First of all, at the end of the day, if the companies intend to deliver quality products, then it becomes an ethical obligation for them to put their equipment through the performance qualification phase first. Other than that, complex equipment often has indiscernible fragile parts, which may take minor damage while in transit from their manufacturer's location to the pharmaceutical plant.
Get ready to use editable Validation Protocols in MS-Word FormatView List


Dear Sir
ReplyDeleteThis blog is very useful and really educated. And I will be more thankful if you can can share PQ protocol sample/template for HVAC and incubator...please send it to:gunawanta.g@supratechnic.co.id
Thankyou
Three reactors are used for same purpose (Hydrolysis). Can I use single Performance Qualification Protocol for the re-qualification of the three reactors? Please suggest.
ReplyDeleteYou you can use same for all but make separate copy for each with their equipment id.
DeleteThank you Sir.
DeleteDear Sir,
ReplyDeleteYour information is very informative and useful for me. Can I ask if you can share some information related to IQ,OQ and PQ for balances using in GMP area, from small scale to large scall. Thanks a lot for your sharing and appreciate for your reply.